Resistance Exercise Improves Spatial Learning Ability Through Phosphorylation of 5’-Adenosine Monophosphate-Activated Protein Kinase in Parkinson Disease Mice
Article information
Abstract
Purpose
Exercise is a representative noninvasive treatment that can be applied to various diseases. We studied the effect of resistance exercise on motor function and spatial learning ability in Parkinson disease (PD) mice.
Methods
The rotarod test and beam walking test were conducted to evaluate the effect of resistance exercise on motor function, and the Morris water maze test was conducted to examine the effect of resistance exercise on spatial learning ability. The effect of resistance exercise on brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) expression and 5’-adenosine monophosphate-activated protein kinase (AMPK) phosphorylation was investigated by Western blot analysis. New cell generation was confirmed by immunohistochemistry for 5-bromo-2’-deoxyuridine.
Results
Resistance exercise improved coordination, balance, and spatial learning ability in PD mice. Resistance exercise enhanced new cell production, BDNF and TrkB expression, and AMPK phosphorylation in PD mice. The effect of such resistance exercise was similar to that of levodopa application.
Conclusions
In PD-induced mice, resistance exercise enhanced AMPK phosphorylation to increase BDNF expression and new neuron generation, thereby improving spatial learning ability. Resistance exercise is believed to help improve symptoms of PD.
• HIGHLIGHTS
- Resistance exercise improved coordination, balance, and spatial learning ability in PD mice.
- Resistance exercise enhanced new cell production, BDNF and TrkB expression, and AMPK phosphorylation in PD mice.
- The effect of such resistance exercise was similar to that levodopa application.
INTRODUCTION
The symptoms of Parkinson disease (PD) are largely divided into impairment of motor function, functional loss of autonomic nervous system, and dysfunction of nonmotor function, and this disorder causes slow movement, tremors, balance problems, and muscle stiffness [1]. From a pathophysiological point of view, dopaminergic neurons present in the substantia nigra are destroyed, and thus dopamine reduction in the striatum is characterized [2]. The cause of dopaminergic apoptosis in the substantia nigra of PD has been reported to be related to increased oxidative stress, mitochondrial dysfunction, and excitotoxicity [3,4], but a clear cause has not been elucidated.
In addition to levodopa, anticholinergic drugs that reduce tremor or drugs that inhibit type B monoamine oxidase are mainly used for the treatment of PD [5]. However, in the case of drug treatment, side effects due to long-term use and dose increase occur frequently, and peripheral disorders such as urination and defecation disorders, and central nervous system disorders such as cognitive dysfunction and delirium have been reported [6,7]. Therefore, for the effective management of PD, it is necessary to establish a treatment plan with few side effects based on the pathophysiological mechanism.
Exercise is a representative noninvasive treatment that can be applied to various diseases. Exercise has a beneficial effect on brain health along with improving cognitive function [8,9]. It was reported that cognitive function improvement through exercise was more effective in the old people [10,11]. Also, regular exercise improved degenerative brain diseases such as stroke, Alzheimer disease, and PD [12,13]. Exercise increased hippocampal size, blood flow, and synaptic plasticity, and neurogenesis [9,12,14]. Although the therapeutic effect of exercise in PD patients is widely known, aerobic exercise is mainly performed rather than resistance exercise due to several limitations. However, resistance exercise is essential to decrease the risk of falls, and it is known that resistance training in PD patients showed improvement in various physical functions [15].
In the current experiment, the effect of resistance exercise on motor function and spatial learning ability in PD-induced mice was studied. The rotarod test and beam walking test were conducted to clarify the effect of resistance exercise on motor function, and the Morris water maze test was conducted to examine the effect of resistance exercise on spatial learning ability. Western blot analysis was done to evaluate the effect of resistance exercise on the brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) expression and 5’-adenosine monophosphate-activated protein kinase (AMPK) phosphorylation. New cell generation was confirmed by immunohistochemistry for 5-bromo-2’-deoxyuridine (BrdU).
MATERIALS AND METHODS
Animals and Grouping
Male ICR mice weighing 30±2 g (10 weeks old) were obtained and prepared for this experiment. The entire process was confirmed by the Animal Ethics Committee of Kyung Hee University, and the following approval number was obtained: KHUSASP (SE)-18-15. The mice were classified into control group, PD group, PD with resistance exercise group, and PD with levodopa group.
PD Induction
PD induction was done as previously explained method [16]. The mice were intraperitoneally injected with 250 mg/kg probenecid diluted in dimethyl sulfoxide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). After 30 minutes injection of probenecid, 20-mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, Sigma-Aldrich Chemical Co.) diluted in 0.9% saline was administered subcutaneously using a 100-μL syringe. All groups except the control group were administered MPTP 10 times during 5 weeks.
Resistance Exercise and Drug Application
The resistance exercise was conducted as previously explained method [16,17]. From the 7th day after MPTP injection, the mice in the exercise group were given ladder-climbing exercise 5 days a week during 5 weeks. The mice were allowed to climb ladder with 30% of their weight on their tails in the first week, 40% of their weight on their tails at 2–3 weeks, and 50% of their weight on their tails at 4–5 weeks. The mice in the PD with levodopa group were orally administered with 25-mg/kg levodopa (100 μL), 5 days a week during 5 weeks.
Rotarod Test
The rotarod test was performed on the 34th day of the first MPTP administration as previously explained method [18,19]. The experimental animals were placed on a rotating cylinder of a rotarod equipment and started at a speed of 4 rpm. Thereafter, the speed of about 4 rpm was gradually increased to reach 45 rpm at 300 seconds, which is the end time of the experiment. The latency until the experimental animals fell from the rotating rotarod device was measured.
Beam Walking Test
A beam walking test was performed on the 35th day of the first MPTP administration as previously explained method [18]. The experimental animals were allowed to walk horizontally on an acrylic rod having a width of 1 cm and a length of 100 cm, and the time it took to move without falling to the end point (100 cm) was measured.
Morris Water Maze Test
Morris water maze test was done as previously explained method [20,21]. For this evaluation, learning started on the 37th day of the first MPTP administration, followed by a 4-day learning period, and then the main test was conducted on the 41st day. A circular pool with a diameter of 140 cm and a height of 45 cm was filled with water (28°C±2°C), and paint of a color symmetrical to the experimental animals was dissolved in water. After training the mice to move from the initial starting point to the destination (diameter, 15 cm; height, 35 cm), when all learning was completed, the destination was removed and the waiting time in the destination area was measured. In all procedures, the movement of the experimental animals was quantified using a Smart Video Tracking System (Smart version 2.5, Panlab, Barcelona, Spain).
Tissue Preparation
After the Morris water maze test was completed, all mice were anesthetized with Zoletil 50 (10 mg/kg, Vibac Laboratories, Carros, France) after which the mice were sacrificed. For fixation in mice, 50mM phosphate-buffered saline and 4% paraformaldehyde were injected via the heart. After brain removal, 40-μm-thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany).
Western Blotting
Western blot analysis for BDNF, TrkB, and AMPK was done as previously explained method [14,22]. Lysis buffer was applied to lyse hippocampal tissues, and protein content was detected by a colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Sodium dodecyl sulfate-polyacrylamide gel was used to separated 30-μg protein, then reaction mixture was transferred to a nitrocellulose membrane, stopped reaction by applying dehydrated milk, and then incubated by primary antibodies. Mouse anti-β actin (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-BDNF (1:1,000; Santa Cruz Biotechnology), rabbit anti-TrkB (1:1,000; Santa Cruz Biotechnology), and rabbit anti-AMPK (1:1,000; Santa Cruz Biotechnology) were selected as the primary antibodies. Horseradish peroxidaseconjugated secondary antibodies were used, and enhanced chemiluminescence detection system (Santa Cruz Biotechnology) measured the expression of bands.
Immunohistochemistry
BrdU immunohistochemistry was done as previous explained method [14,22]. BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany) was applied to the sections overnight at 4°C. Biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) was treated to the sections for 1 hour and ABC complex (1:100; Vector Laboratories) was applied for 1 hour. In order to visualize, the sections were treated to 50mM Tris-HCl (pH, 7.6) with 0.03% diaminobenzidine, then 0.03% H2O2, and 40-mg/mL nickel chloride for 5 minutes.
Data Analysis
The number of BrdU-positive cells in the hippocampal dentate gyrus was measured from the hemilateral side and presented as the cell number/mm2. The detected bands from the Western blot were counted using a densitometer. For data analysis, 1-way analysis of variance and Duncan post hoc test was conducted and the results were presented as mean±standard error of the mean. P<0.05 showed statistical significance.
RESULTS
Coordination and Balance
The induction of PD reduced latency in rotarod test and increased arrival time in beam walking test compared to the control group. Meanwhile, resistance exercise extended waiting time in rotarod test and reduced arrival time in beam walking test, and had a similar effect to the group treated with levodopa (Fig. 1).
Spatial Learning Ability
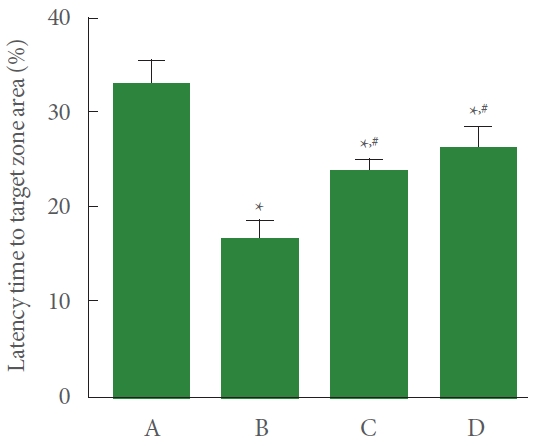
The induction of PD shortened the time spent at the target zone compared to the control group. Meanwhile, resistance exercise lengthened the time spent at the target zone, and had a similar effect to the group treated with levodopa (Fig. 2).
New Cell Generation
The induction of PD suppressed the number of BrdU-positive cells in the hippocampal dentate gyrus compared to the control group. Meanwhile, resistance exercise potentiated the number of BrdU-positive cells, and had a similar effect to the group treated with levodopa (Fig. 3).
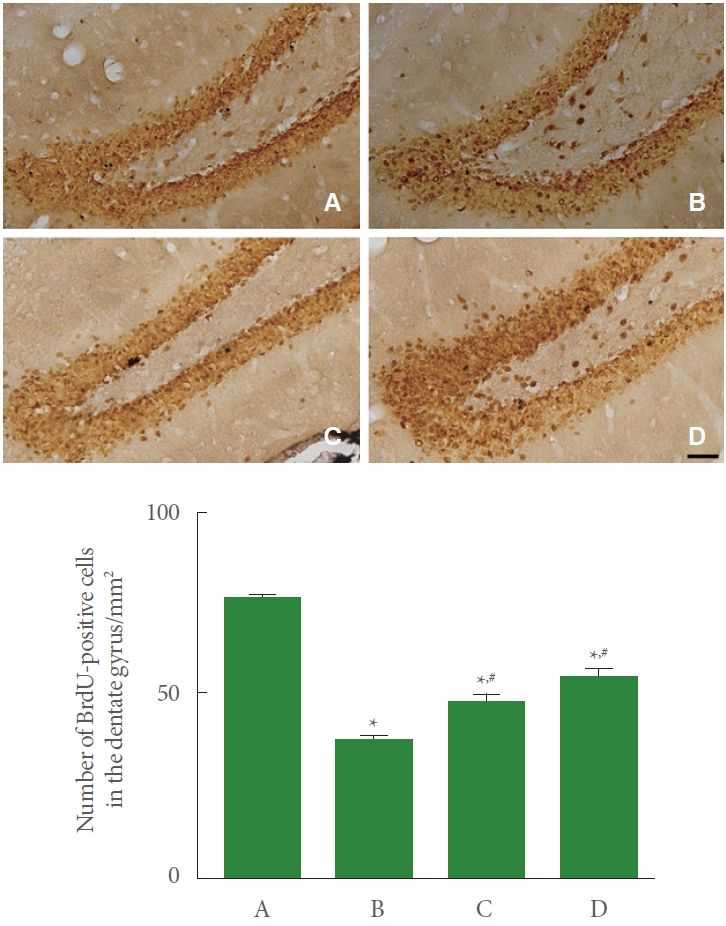
Effect of resistance exercise on cell proliferation in the hippocampus. Upper panel: photomicrographs of 5-bromo-2’- deoxyuridine (BrdU)-positive cells. The scale bar represents 150 μm. Lower panel: number of BrdU-positive cells in each group. A, control group; B, Parkinson disease (PD) group; C, PD with resistance exercise group; D, PD with levodopa group. *P<0.05 compared to the control group. #P<0.05 compared to the PD group.
BDNF and TrkB Expression
The induction of PD reduced BDNF and TrkB expression in the hippocampus compared to the control group. Meanwhile, resistance exercise increased BDNF and TrkB expression, and had a similar effect to the group treated with levodopa (Fig. 4).
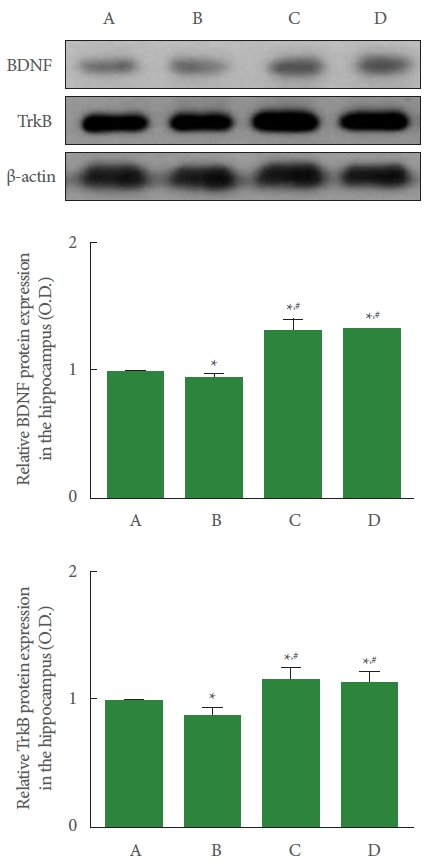
Effect of resistance exercise on brain-derived neurotrophic factor (BDNF) and tropomyosin kinase B (TrkB) expression in the hippocampus. Upper panel: representative expression of BDNF and TrkB. Middle panel: relative expression of BDNF in each group. Lower panel: relative expression of TrkB in each group. A, control group; B, Parkinson disease (PD) group; C, PD with resistance exercise group; D, PD with levodopa group. *P<0.05 compared to the control group. #P<0.05 compared to the PD group.
AMPK Phosphorylation
The induction of PD reduced the ratio of AMPK phosphorylation in the hippocampus compared to the control group. Meanwhile, resistance exercise enhanced ratio of AMPK phosphorylation, and had a similar effect to the group treated with levodopa (Fig. 5).

Effect of resistance exercise on phosphorylation of adenosine monophosphate protein kinase (AMPK) in the hippocampus. Upper panel: representative expression of AMPK. Lower panel: ratio of AMPK to phosphorylated AMPK (pAMPK) in each group. A, control group; B, Parkinson disease (PD) group; C, PD with resistance exercise group; D, PD with levodopa group. *P<0.05 compared to the control group. #P<0.05 compared to the PD group.
DISCUSSION
Traumatic brain-injured rats showed obvious neurological deficits with decreased latency in the rotarod test and increased arrival time in the beam gait test [18]. MPTP-induced Parkinsonism showed motor deficits in behavioral tests including beam walking test and pole test [23]. Swimming exercise improved motor coordination and balance in the rotarod test and vertical pole test of the experimental autoimmune encephalomyelitis rats [19]. Treadmill running improved motor function in the rotarod test and foot fault test of the photothrombotic stroke mice [24]. In the results of this experiment, the induction of PD decreased coordination and balance in the rotarod test and beam walking test compared to the control group. However, resistance exercise improved coordination and balance, and the effect was similar to that of levodopa treatment.
MPTP-induced PD mice showed impairment of spatial learning ability in the Morris water maze test with tyrosine hydroxylase-positive neuronal loss [25]. Impairment of motor skills in PD patients is associated with cognitive impairment. PD patients with mild cognitive impairment performed significantly worse in accuracy, dexterity, and speed (arm hand movements) compared to the PD patients without mild cognitive impairment [26]. Treadmill running improved short-term memory in step-through avoidance task in the photothrombotic stroke mice [24]. In the results of this experiment, the induction of PD impaired spatial learning ability in Morris water maze test compared to the control group. However, resistance exercise improved spatial learning ability, and the effect was similar to that of levodopa treatment.
There are many reports that exercise enhances newly generated neurons in the hippocampus. Treadmill running enhanced new cell production in the hippocampal dentate gyrus of oldaged rats [11]. Reduced hippocampal neurogenesis was observed in the 6-hydroxydopamine-induced PD rat model [27]. Treadmill running increased short-term memory by enhancing the production of new neurons in rat pups born to obese mother rats [14]. Treadmill running exerted a potentiating effect on synaptic plasticity and new cell generation in the hippocampus of stroke mice [24]. In the results of this experiment, the induction of PD inhibited new cell formation in the hippocampal dentate gyrus compared to the control group. However, resistance exercise potentiated new cell production, and the effect was similar to that of levodopa treatment.
BDNF and its receptor TrkB, which are most abundantly expressed in the brain, modulate axonal and dendritic growth in the nervous system, and they play important roles in learning and memory process [28]. Dysfunction of striatal neurons is a major cause of motor impairment in Huntington’s disease, and BDNF supports the promotion of survival, maturation, and connectivity of striatal neurons during brain development [29]. Treadmill running enhanced BDNF and TrkB expression in the hippocampus of rat pups born to obese maternal rats [14]. In mice with photothrombotic stroke, a potentiating effect of treadmill running on hippocampal neuronal production by increasing hippocampal BDNF and TrkB expression was shown [24]. Treadmill running ameliorated short-term and spatial working memory impairment though increment of BDNF and TrkB expression in socially isolated rats [30]. In the results of this experiment, the induction of PD suppressed the expression of BDNF and TrkB in the hippocampus compared to the control group. However, resistance exercise increased expression of BDNF and TrkB, and the effect was similar to that of levodopa treatment.
AMPK is a heterotrimeric protein complex made by α, β, and γ subunits and is the serine/threonine kinase that modulates cellular energy homeostasis. When the α subunit of AMPK is phosphorylated, AMPK is activated [31]. In exercising muscle cells, AMPK increased blood supply by stimulating angiogenesis [32]. AMPK activation produced an antidepressant effect, which was potentially mediated by hippocampal neurogenesis via BDNF/TrkB signaling in neurons [33]. Increased AMPK phosphorylation by voluntary wheel running in aged rats improved vascular endothelial growth factor expression and alleviated sarcopenia [34]. In the results of this experiment, the induction of PD suppressed AMPK phosphorylation in the hippocampus compared to the control group. However, resistance exercise enhanced AMPK phosphorylation, and the effect was similar to that of levodopa treatment.
In the current study, resistance exercise in PD-induced mice enhanced AMPK phosphorylation to increase BDNF expression and new neuron generation, thereby improving spatial learning ability. Resistance exercise is believed to help improve symptoms of PD.
Notes
Fund/Grant Support
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018S1A5A2A01036668).
Research Ethics
The experimental procedure was approved by the Kyung Hee University Animal Ethics Committee and received approval number KHUASP (SE)-18-15.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
• Conceptualization: SHK
• Data curation: LH, JJJ, IGK
• Formal analysis: LH, JJJ, IGK
• Funding acquisition: SSB
• Methodology: LH, JJJ, IGK
• Project administration: SSB
• Visualization: YBK, HSY
• Writing-original draft: SHK
• Writing-review & editing: SHK