Efficacy and Safety of Low-Dose Intravesical OnabotulinumtoxinA Injections in Female Patients With Detrusor Overactivity With Detrusor Underactivity
Article information
Abstract
Purpose
We assessed the effectiveness and safety of using intravesical onabotulinumtoxinA (onabotA; BOTOX) injection with a low dose (75 units) for treating urinary storage symptoms in patients with detrusor overactivity with detrusor underactivity (DODU) compared to using the standard 100 units of onabotA in patients with overactive bladder (OAB).
Methods
This ambidirectional study included 121 female patients who received intravesical onabotA injections at our hospitals. A total of 87 patients with OAB and 34 patients with DODU were reviewed using a 3-day voiding diary, uroflowmetry, and questionnaires including the International Prostate Symptom Score (IPSS), Overactive Bladder Symptom Score, and Patient Perception of Bladder Condition. Patients were evaluated at baseline, within 2 weeks of treatment, and beyond 3 months after treatment.
Results
Questionnaire scores of the DODU group demonstrated significant improvement in the short term, with a subsequent decline, but an overall improvement compared to baseline in the long term. Notably, the DODU group exhibited enhanced IPSS voiding scores after the treatment. In the OAB group, most questionnaire scores, excluding the IPSS voiding score, showed significant posttreatment improvement, which was sustained to some extent in the long term. Voiding diary parameters related to storage symptoms were enhanced in both groups. The maximum and mean flow rates decreased in the OAB group but increased in the DODU group, particularly in the short term (P=0.000). The postvoid residual volume increased in both groups after posttreatment, with a mitigated change in the long term. Safety assessments revealed manageable adverse events in both groups with comparable frequencies.
Conclusions
Low-dose intravesical onabotA for DODU demonstrated a relatively shorter duration of efficacy than OAB. Nonetheless, the treatment improved both storage and voiding symptoms in patients with DODU without significant adverse effects.
INTRODUCTION
Overactive bladder (OAB) is a common condition characterized by urgency, with or without urinary incontinence, increased daytime frequency, and nocturia [1]. Underactive bladder (UAB) is a symptom complex suggestive of detrusor underactivity (DU), resulting in prolonged or incomplete bladder emptying [1]. Detrusor overactivity with DU (DODU) is a condition in which the bladder is both overactive and underactive, resulting in storage symptoms, such as urgency urinary incontinence (UUI) and voiding difficulties [2]. DODU is a common cause of urinary incontinence in elderly patients and must be diagnosed to avoid unnecessary interventional management.
There is no defined treatment algorithm for patients with DODU; however, they are typically treated based on symptom severity [3]. Treatments for patients with voiding symptoms include alpha-blockers or clean intermittent catheterization (CIC); sacral nerve stimulation may also be considered. For patients with storage symptoms, the first-line treatment is behavioral therapy, such as fluid intake restriction, timed voiding, and Kegel exercises. When behavioral therapy is insufficient, oral medications, including antimuscarinic agents or beta-3 adrenoreceptor agonists are the primary second-line treatment. Highly resistant storage symptoms refractory to oral medications are treated with an intravesical injection of onabotulinumtoxinA (onabotA; BOTOX, Allergan plc, Dublin, Ireland) as an effective third-line treatment [4,5]. Complications of intravesical injection of onabotA include increased postvoid residual (PVR) volume, acute urinary retention (AUR), and risk of urinary tract infection (UTI) [6]. These complications can result in poor patient compliance. Thus, appropriate patient selection is crucial, and patients who are willing to undergo PVR evaluation and CIC are appropriate candidates.
Intravesical onabotA injections can also be used to treat storage symptoms in patients with DODU; however, only one clinical study has been conducted on this topic to date [7]. We conducted our research to assess the effectiveness and safety of intravesical onabotA injection at a low dose of 75 units (U) for treating storage symptoms in patients with DODU compared to the standard 100 U of onabotA in patients with OAB.
MATERIALS AND METHODS
Study Design
This ambidirectional study involved patients who received intravesical onabotA injections at the 2 authors’ hospitals between 2017 and 2022. All enrolled patients had to be female, between 20 and 80 years of age and had had at least one episode of urgency or UUI per day, as recorded in a 3-day voiding diary. Although there is currently no consensus on the definitions of DU and DODU [8-10], very strict criteria have been suggested based on expert opinions of the European Association of Urology (EAU) [11]. Women with DU were defined as having detrusor pressure at a maximum flow rate <20 cm H2O, maximum flow rate (Qmax) <15 mL/sec, bladder voiding efficiency (BVE) <90%, and no signs of obstruction on video-urodynamic studies. Based on this definition, patients with DODU are required to fulfill these criteria.
The enrolled patients had tried behavioral therapy and medication with antimuscarinics for more than 3 months before enrollment. All patients stopped antimuscarinics for more than 2 weeks before the injection to wash out the residual effect. The exclusion criteria were neurogenic bladder dysfunction, bladder outlet obstruction, malignant diseases of the pelvic organs, UTI, and other serious diseases that made the patient unsuitable for the study. OnabotA was injected transurethrally using a cystoscope and a 23-gauge needle. Patients were treated with suburothelial injections of onabotA in 10-mL saline, 0.5 mL per injection in 20 injections in the bladder body, sparing the trigone, as a recent randomized clinical trial showed that trigone injections are not superior to trigone-sparing injections in efficacy but only increased the incidence of UTI and voiding difficulty [12]. Patients with OAB were treated with 100-U onabotA, whereas those with DODU were treated with 75-U onabotA.
Efficacy and Safety Assessments
A 3-day voiding diary, uroflowmetry, and questionnaires including the International Prostate Symptom Score (IPSS), Overactive Bladder Symptom Score (OABSS), and Patient Perception of Bladder Condition were reviewed to assess the efficacy of intravesical onabotA injections. Patients were evaluated at baseline, within 2 weeks of treatment, and beyond 3 months after treatment. Procedure-related complications were monitored. Common adverse events recorded during the follow-up period after onabotA treatment included AUR, PVR volume >200 mL, gross hematuria, general weakness, and UTI. Patients who developed AUR or PVR volumes of >350 mL were treated with Foley catheter insertion or advised to undergo CIC to empty their bladders as needed.
Statistical Analysis
Statistical comparisons of demographics and safety assessments between the groups were conducted using the chi-square test for categorical variables and the independent t-test for continuous variables. A linear mixed model was employed to determine the statistical significance of treatment efficacy between the DODU and OAB groups. Analysis of variance was used to compare the parameters before and after treatment. Statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient Characteristics
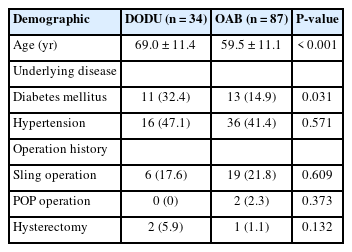
In total, 121 patients who received intravesical onabotA injections were selected. Of these, 87 had OAB, and 34 had DODU. All the enrolled patients were female and aged between 20 and 80 years. The mean age±standard deviation of the DODU and OAB patients were 69.0±11.4 years and 59.5±11.1 years, respectively. There were statistical differences in age and presence of diabetes mellitus (DM) between the 2 groups. No significant differences in other demographic data were observed between the 2 groups (Table 1).
Efficacy Assessment
In the DODU group, all questionnaire scores demonstrated more dramatic and statistically significant improvements in the postoperative ≤2-week period compared to the OAB group (Table 2). In the postoperative ≥12-week period, there was a subsequent decline from the initial improvement; however, an overall trend of improvement compared to baseline was observed. Notably, the DODU group showed an improvement in the IPSS voiding score after onabotA treatment. In the OAB group, most questionnaire scores, excluding the IPSS voiding score, showed significant improvement after onabotA treatment, and this effect persisted to some extent in the postoperative ≥12-week period. The P-values derived from the linear mixed model between the DODU and OAB groups for IPSS voiding and total scores were 0.016 and 0.021, respectively.
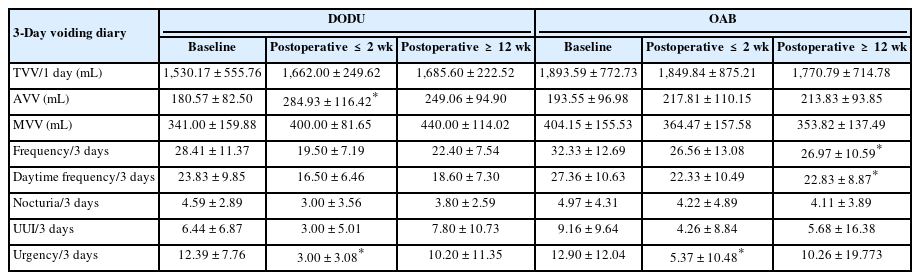
Time-course changes in the voiding diary parameters are presented in Table 3. Both urgency and UUI showed substantial improvements in the postoperative ≤2-week period in both groups, with relatively modest effects in the postoperative ≥12-week period (P=0.936 and P=0.460, respectively). While the frequency, daytime frequency, and nocturia parameters also improved in both groups, there was a prominent increase in the average voided volume (AVV) in the DODU group compared to that in the OAB group (P=0.489). The maximum voided volume (MVV) also showed improvement in the DODU group, whereas no improvement was observed in the OAB group (P=0.357).
As shown in Table 4, the Qmax and mean flow rate values decreased in the OAB group after onabotA treatment, whereas the DODU group exhibited increased values, with a more pronounced effect observed during the postoperative ≤2-week period. P-values derived by the linear mixed model between the DODU and OAB groups for Qmax and mean flow rate were 0.000. The increase in the voided volume (VV) was more prominent in the DODU group than in the OAB group (P=0.104). The PVR volume increased in both groups posttreatment, with a mitigated rate of change in the postoperative ≥12-week period (P=0.132).
Safety Assessment
Postoperative complications after onabotA treatment are presented in Table 5. AUR occurred in one patient in each group, and both cases were managed with CIC (P=0.487). A high PVR exceeding 200 mL was more prevalent in the OAB group than in the DODU group, although the difference was not statistically significant (17.2% vs. 8.8%, respectively; P=0.242). Dysuria and a weak stream were reported by patients with OAB (10.3% and 5.7%, respectively), but not by patients with DODU. The frequencies of other adverse events were comparable between the groups.
DISCUSSION
DU is characterized by reduced strength and/or duration of bladder contractions, resulting in prolonged bladder emptying or failure to achieve complete bladder emptying within a normal period, as defined by the International Continence Society [13]. However, the definitions of “prolonged bladder emptying” and “normal period” lack clarity, leading to varying definition interpretations of DODU in clinical studies [8-10]. Wang et al. [7] defined DODU as a baseline PVR volume of >100 mL but <250 mL or a BVE of <67% in patients with OAB. Despite this inconsistency, a comprehensive urodynamic database study utilized a stringent definition based on EAU expert opinions [11]; women with DU were defined as those with PdetQmax <20 cm H2O, Qmax <15 mL/sec, BVE <90%, and no sign of obstruction in video-urodynamic studies. Our study adhered to these criteria when selecting patients for DODU.
Intravesical onabotA 100 U is the standard dose for treating refractory idiopathic OAB in adults of both sexes and is licensed in Europe [14,15]. However, elderly and fragile patients with OAB may experience advantages from a lower dosage of onabotA owing to alterations in bladder physiology associated with aging, increased usage of medications affecting detrusor function, and a reciprocal connection with additional geriatric conditions such as frailty, rendering a lower dosage more suitable for them [16].
Despite numerous studies investigating the appropriate dosage for onabotA treatment in OAB [14-16], there is a lack of established optimal dosages for onabotA in DODU treatment, and limited clinical investigations of its efficacy and safety. To address this gap, our study explored the use of a lower dosage, specifically 75 U, for treating DODU, considering the potential exacerbation of voiding symptoms after Botox therapy due to concurrent voiding impairment in these patients. The results indicated a shorter duration of treatment compared to OAB, yet a notable improvement in the storage symptoms of DODU was achieved without significant complications. This improvement was observed not only in subjective surveys but also in voiding diaries, where parameters related to storage symptoms showed enhancement.
The efficacy of the onabotA therapy established for OAB is hypothesized to apply to DODU because of its shared pathophysiological characteristics. Morphological analysis of bladder biopsies from patients with DODU has demonstrated characteristics of both detrusor overactivity (dysjunctional pattern) and impaired detrusor contractility typically observed in aging bladders (neural degeneration, interstitial fibrosis, and dense band pattern in smooth muscle cells) [2,17]. The hypothesis that chronically untreated or treatment-refractory OAB can progress over time to DODU and subsequently to UAB provides further support [18]. Therefore, the mechanism of action of onabotA may also apply to DODU. OnabotA inhibits the release of neurotransmitters such as acetylcholine and adenosine triphosphate at presynaptic cholinergic junctions, restrains detrusor contraction, and augments bladder capacity [19,20].
A noteworthy observation in our study was the improvement in the IPSS voiding score, Qmax, and mean flow rate following onabotA treatment for DODU. This trend suggests that the onabotA effect of reducing bladder sensitivity and increasing bladder capacity leads to larger VVs, thereby potentially affecting voiding symptoms and flow rate. Consequently, the increase in VV, AVV, and MVV after onabotA treatment was more pronounced in the DODU group compared to the OAB group, as observed in this study. This finding is consistent with that of a previous investigation of mirabegron in female patients with OAB. Matsukawa et al. reported that mirabegron not only improved storage function but also increased voiding function parameters, such as Qmax and PdetQmax [21].
Conversely, our observations differ from a previous study by Wang et al. [7], the only clinical study reporting the impact of intravesical onabotA injection in patients with DODU to date, in which Qmax decreased after treatment in patients with DODU. Wang et al. [7] reported that, although 100 U of intravesical onabotA injection improved subjective urgency symptoms without increasing the risk of adverse events in patients with DODU, voiding diary parameters, such as the number of urgency episodes, frequency, or UUI, and uroflowmetry parameters did not show improvement after treatment. This discrepancy could be attributed to differences in the diagnostic criteria for DODU and variations in the onabotA dosage.
Limitations of this study include its ambidirectional design, potential bias, and limited generalizability. Although there were no significant differences in other demographic data, the higher mean age and increased prevalence of DM in the DODU group than in the OAB group were recognized as limitations. We employed a general linear model to test the study results and mitigate the potential confounding effects of age and diabetes prevalence. Most parameters did not show a statistically significant relationship, except for OABSS and diabetes prevalence, which had a P-value of 0.010, and Qmax and age, which had a P-value of 0.045. Consequently, the 2 groups can be considered homogeneous, except for the inherent characteristics of the diseases, making it meaningful to compare the effects of intravesical onabotA injections in each group. In addition, the relatively small sample size emphasizes the need for larger studies to corroborate these findings. A randomized dose-ranging trial to compare different doses of onabotA in patients with DODU should be conducted in future studies.
To the best of our knowledge, this is the first study to report the efficacy and safety of low-dose intravesical onabotA treatment for DODU. Low-dose intravesical onabotA treatment for DODU demonstrated a relatively shorter duration of efficacy than that for OAB. Nonetheless, the treatment improved both storage and voiding symptoms in patients with DODU without significant adverse effects. These findings highlighted the potential of onabotA as a treatment option for patients with DODU.
Acknowledgements
We appreciate the in-depth review of the statistical and methodological accuracy of the Academic Clinical Research Operating and Supporting System of Chungnam National University Hospital Biomedical Research Institute.
Notes
Grant/Fund Support
This study was supported by the Chungnam National University Hospital Research Fund, 2021.
Research Ethics
This study was approved by the Institutional Review Board (IRB) of Chungnam National University Hospital (IRB-2021-03-078-005) on March 8, 2021, and Chungnam National University Sejong Hospital (IRB-2021-09-011) on October 1, 2021.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: HSN, CLL, JHS
· Data curation: HSN, JSL, KHS, JHS, JMP
· Formal analysis: HSN, CLL
· Funding acquisition: HSN, JYL
· Methodology: HSN
· Project administration: HSN, JHS, JYL
· Visualization: HSN
· Writing - original draft: HSN
· Writing - review & editing: HSN, CLL, JHS, JYL